Selected Publications
For a complete list of publications, click here
Magnetic particle imaging: The need for standardized approaches
Poptani H., O'Brien, L., Giardiello, M.
Matter, (2024). doi.org/10.1016/j.matt.2024.05.049

Magnetic particle imaging (MPI) represents a growing area for materials discovery toward the design of magnetic tracers for real-time, functional, and quantitative medical imaging. The work conducted between the University of Florida, USA (Rinaldi-Ramos), and The Robarts Research Institute at Western University, Canada (Foster), highlights the need for unified analytical approaches in order to optimize MPI tracer development, outlined in their paper published in Molecular Imaging and Biology.
Controllable and tuneable growth of NaYbF4:Tm (0.5%) Fe (5%) @ Na(Yb/Y)F4 - core @ shell and the effect of their geometry on upconversion luminescence.
Ureña-Horno, E., Liu K., Giardiello, M.
Journal of Materials Chemistry C, (2023). doi.org/10.1039/D3TC01215H

Lanthanide doped Upconversion Nanoparticles (UCNPs) are a class of nanomaterials with excellent luminescence properties. The practical use of UCNPs, however, has been hindered by their relatively low upconversion (UC) quantum yields. Enhancing this efficiency, and therefore their brightness, is a critical goal for this emerging material. In this study, a range of novel core @ shell structures were synthesized using NaYbF4:Tm(0.5%) Fe(5%) as the core template to regulate the shell growth of Na(Yb/Y)F4. Here, we observed that the size and the morphology of the UCNP can be fine-tuned by controlling the ratio of the Y3+/Yb3+ ions within the shell material, resulting in small rod-like structures that form when using a high concentration of Y3+ and larger hexagonal plate-like structures when using a high concentration of Yb3+. In terms of the optical properties, the UC luminescence and lifetime of the core-only and core @ shell structures were measured. Overall, the emission intensity and lifetime of the UCNPs increased with the nanoparticle size. We observed that, under the same experimental conditions, larger core @ shell nanostructures with hexagonal shape showed brighter UC emission, compared to those with small nanorod shape and core-only. Based on our findings, we propose varied of energy transfer pathways to arise through geometric alteration of the nanostructures upon varied Y3+/Yb3+ ions concentration ratios within the shell material. Our results could have important significance for understanding the relationship between the geometry of UCNPs and their UC optical properties.
Aqueous (co)polymer stabilisers for size-controlled 2–5 nm gold nanoparticle synthesis with tuneable catalytic activity
Traynor, D., Ureña-Horno, E., Hobson, J. J., Croft, E. J., Edwards, S. E., Rannard, S. P., Giardiello, M.
New Journal of Chemistry, 46, 17282-17291 (2022). doi: 10.1039/d2nj03257k

Gold nanoparticles, or colloidal gold (AuNP), represent one of the most significant and established forms of sub-micron inorganic structures to be researched in recent years. AuNP physical and chemical properties are dictated by both their ligand surface chemistry and their size, which can be manipulated and tuned during their synthesis. In this study, aqueous linear and branched homo-polymers and (co)polymers are developed and used as surface stabilisers during AuNP synthesis. A library of such polymeric stabilisers were prepared using conventional free radical polymerisation techniques to incorporate units of varying AuNP surface binding affinity, using methacrylic acid (MAA) and oligo (ethylene glycol) methyl ether methacrylate (OEGMA) monomers and dodecane thiol (DDT) as the chain transfer agent. AuNPs were synthesised via HAuCl4·3H2O reduction in the presence of the prepared library of polymeric stabilisers. It was observed that variation of (co)polymer composition and architecture allowed for size-controlled gold nanoparticle synthesis, with AuNPs prepared ranging from 2.17 ± 0.07 nm to 4.83 ± 0.04 nm as determined by UV-vis spectroscopy. Varying (co)polymer composition and architecture also yielded variable catalytic behaviour in the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) using NaBH4, with catalytic reaction rates ranging from 1.0 s−1 to 45.3 s−1 and induction times ranging from 0 seconds to 2070 seconds depending on the polymeric stabilisers employed during synthesis.
Stable, polymer-directed and SPION-nucleated magnetic amphiphilic block copolymer nanoprecipitates with readily reversible assembly in magnetic fields
Giardiello, M., Hatton, F. L., Slater, R. A., Chambon, P., North, J., Peacock, A. K., He, T., McDonald, T. O., Owen, A., Rannard, S. P.
Nanoscale, 8, 7224-7231 (2016). doi.org/10.1039/C6NR00788K
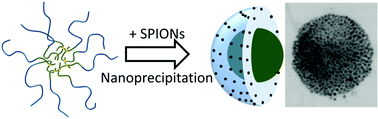
The formation of inorganic–organic magnetic nanocomposites using reactive chemistry often leads to a loss of super-paramagnetisim when conducted in the presence of iron oxide nanoparticles. We present here a low energy and chemically-mild process of co-nanoprecipitation using SPIONs and homopolymers or amphiphilic block copolymers, of varying architecture and hydrophilic/hydrophobic balance, which efficiently generates near monodisperse SPION-containing polymer nanoparticles with complete retention of magnetism, and highly reversible aggregation and redispersion behaviour. When linear and branched block copolymers with inherent water-solubility are used, a SPION-directed nanoprecipitation mechanism appears to dominate the nanoparticle formation presenting new opportunities for tailoring and scaling highly functional systems for a range of applications.
Accelerated oral nanomedicine discovery from miniaturized screening to clinical production exemplified by paediatric HIV nanotherapies
Giardiello, M., Liptrott, N. J., McDonald, T. O., Martin, P., Smith, D., Rannard S.P., Owen, A.
Nature Communications, 7, article number 13184, (2016) doi:10.1038/ncomms13184

Considerable scope exists to vary the physical and chemical properties of nanoparticles, with subsequent impact on biological interactions; however, no accelerated process to access large nanoparticle material space is currently available, hampering the development of new nanomedicines. In particular, no clinically available nanotherapies exist for HIV populations and conventional paediatric HIV medicines are poorly available; one current paediatric formulation utilizes high ethanol concentrations to solubilize lopinavir, a poorly soluble antiretroviral. Here we apply accelerated nanomedicine discovery to generate a potential aqueous paediatric HIV nanotherapy, with clinical translation and regulatory approval for human evaluation. Our rapid small-scale screening approach yields large libraries of solid drug nanoparticles (160 individual components) targeting oral dose. Screening uses 1 mg of drug compound per library member and iterative pharmacological and chemical evaluation establishes potential candidates for progression through to clinical manufacture. The wide applicability of our strategy has implications for multiple therapy development programmes.
pH-Responsive Lanthanide Complexes Based on Reversible Ligation of a Diphenylphosphinamide
Giardiello, M., Botta, M., Lowe, M. P.,
Inorganic Chemistry, 52 (24), 14264 – 14269 (2013) doi.org/10.1021/ic402205j
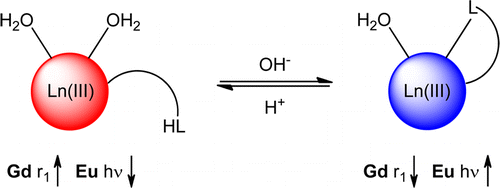
Ln-dpp-DO3A-based complexes [dpp is a pendant diphenylphosphinamide moiety and DO3A is 1,4,7-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecane] exhibit pH-responsive reversible ligation of the phosphinamide for both the gadolinium(III) and europium(III) analogues. pKa values were 8.1 (±0.1) and 7.8 (±0.1) for Gd.1 and Gd.2, respectively. The relaxivities (20 MHz, 298 K) of the gadolinium(III) analogues were r1 = 7.9 mM–1 s–1 (Gd.1) and r1 = 8.2 mM–1 s–1 (Gd.2) in acidic media, corresponding to a hydration state q = 2; in basic media, deprotonation and coordination of the phosphinamide occurs, with r1 = 5.4 mM–1 s–1 (Gd.1) and r1 = 4.4 mM–1 s–1 (Gd.2) corresponding to q = 1. Sensitized luminescent emission was observed from the europium(III) analogues following excitation at λex = 270 nm. The hydration state values of the europium(III) analogues were consistent with those of the gadolinium(III) complexes, i.e., q = 1 and 2 in basic and acidic media, respectively. The ratio of the emission intensities of the ΔJ = 1 and 2 bands enables concentration-independent reporting of the pH. Excited-state pKa values were 8.3 (±0.1) and 8.5 (±0.1) for Eu.1 and Eu.2, respectively.
Facile synthesis of complex multi-component organic and organic-magnetic inorganic nanocomposite particles
Giardiello, M., McDonald, T. O., Smith, D., Martin, P., Owen, A., Rannard, S.P.
Journal of Materials Chemistry, 22 (47), 24744 - 24752 (2012) doi.org/10.1039/C2JM34974D
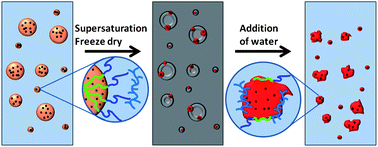
A generic in situ method for producing triple component hydrophobic inorganic–organic nanocomposite particles, using a combination of modified emulsion templating and freeze-drying, is presented. Model nanocomposite particles have been developed bearing up to three hydrophobic ingredients chosen from polystyrene, oil red and 15–20 nm oleic acid-coated super-paramagnetic iron oxide (Fe3O4) nanoparticles. The technique avoids harsh conditions, in situ polymer synthesis and lengthy workup procedures, and results in high incorporation of magnetic particles (approximately 80% of triple-component nanocomposite particles contain magnetite) with retention of super-paramagnetism (>90% preservation). The nanocomposites have been characterised using dynamic light scattering, and studied under static and flow conditions in the presence of magnetic fields. Drug release was demonstrated using model nanocomposite particles bearing ibuprofen with differing hydrophobic polymer; polycaprolactone and poly(n-butyl methacrylate). Drug release varied with temperature, suggesting the synthetic technique could thus be adopted to develop drug carrier particles with tailored drug release properties.
Luminescence Study of Eu(III) Analogues of Esterase-Activated Magnetic Resonance Contrast Agents
Giardiello, M. Lowe, M. P.,
Inorganic Chemistry 48 (17), 8515 – 8522 (2009) doi.org/10.1021/ic901284t
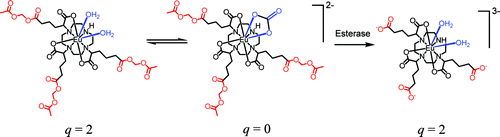
A model for an accumulation and enzyme-activation strategy of a magnetic resonance contrast agent was investigated via the luminescence of Eu(III) analogues. Neutral q = 2 Eu(III) ethyl and acetoxymethyl ester LnaDO3A-based complexes showed increased emission intensity in the presence of serum concentrations of carbonate because of inner-sphere water molecule displacement by the anion. The affinity for carbonate is suppressed by the introduction of negative charge to the complex following enzymatic hydrolysis of the ester groups, resulting in quenching of Eu(III) luminescence and changes in spectral form. The conversion of neutral, carboxylic ester-containing complexes into free acid forms by enzymatic hydrolysis using pig liver esterase was demonstrated by luminescence (Eu) and 1H NMR spectroscopic investigations (Y). These studies demonstrated that the concept of inhibition of anion binding as a result of enzyme activation is feasible.
An esterase-activated magnetic resonance contrast agent
Giardiello, M., Lowe, M. P., Botta, M.,
Chemical Communications, 4044 – 4046 (2007) doi.org/10.1039/B711989E
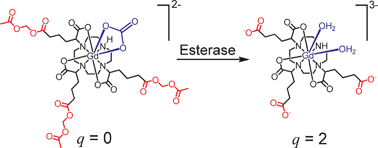
A Gd(III) complex bearing pendant acetoxymethyl esters is activated on exposure to porcine liver esterase; the 84% increase in relaxivity is a result of suppression of HCO3−/CO32− binding by the resulting negative charge
